Cell Filling System
Cell Filling System
AbioFill V100 Bringing High-Throughput Cell Filling into the Automation Era
• Automated operation for effortless large-scale cell filling
• Flexible configuration with filling volume ranging from 250 μL to 5 mL
• Highly efficient: easily dispense ~1,000 cryovials in 30 minutes
• GMP compliant with audit trails and multi-level permission management
• High consistency with a filling volume accuracy error of less than 5%
• Designed for the automated filling of cell banks, strain libraries, and plasmid libraries
The AbioFill V100 is an automated filling system designed for the fast and automatic uncapping, filling, and recapping of cryovials. It minimizes the exposure time of samples to cryoprotectants and reduces the contamination risk associated with repetitive manual operations. This system is widely used for creating higher-quality and more consistent cell banks, strain libraries, plasmid libraries, and the automated filling of biological products
Key Features & Benefits
• Automated operation for effortless large-scale cell filling
• Flexible configuration with filling volume ranging from 250 μL to 5 mL
• Highly efficient: easily dispense ~1,000 cryovials in 30 minutes
• GMP compliant with audit trails and multi-level permission management
• High consistency with a filling volume accuracy error of less than 5%
• Designed for the automated filling of cell banks, strain libraries, and plasmid libraries
Cell filling process
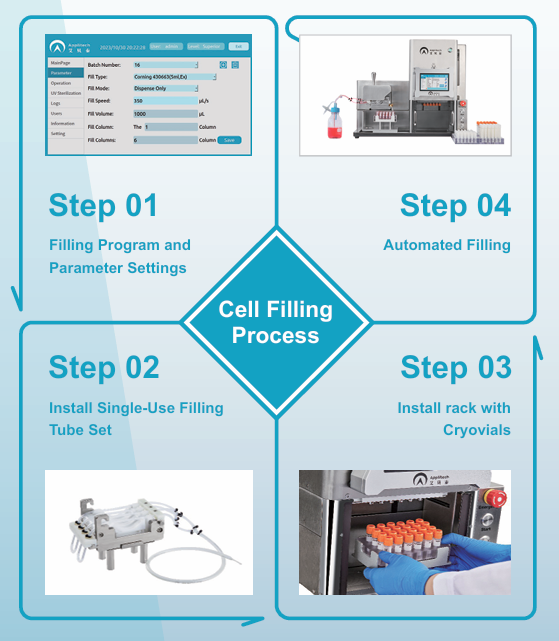
Single-Use Sterile Filling Consumables to Prevent Cross-Contamination
The use of single-use sterile filling consumables eliminates the risk of cross-contamination. The filling tube set is packaged in double-layer sterile bags and sterilized by irradiation. The left side of the filling tube can be connected to shaker tubes, shake flasks, or sterile Wave bags, offering flexible usage options.

Simple Installation


Fast and Gentle Automated Filling Process with Excellent Consistency
• Compared to traditional manual operations, the automated filling system simplifies and acceler ates the filling process, enabling the dispensing of ~1,000 cryovials in just 30 minutes.

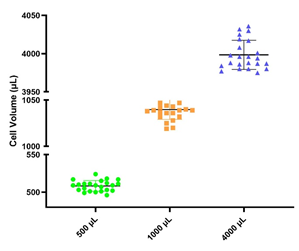
• Within the filling range, 24 cryovials were filled with volumes of 500 μL, 1,000 μL, and 4,000 μL, achieving a volume accuracy error of less than 5%.
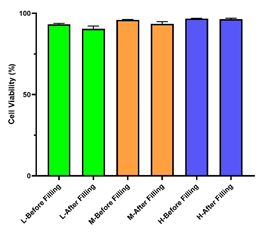
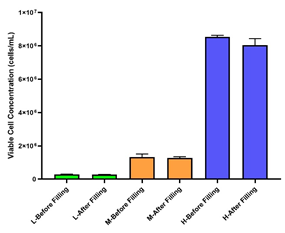
• Three different densities of CHO cells (high, medium, and low) were filled into 24 cryovials. The deviation in cell viability before and after filling was less than 2%, and the deviation in viable cell density was less than 5%. The AbioFill V100 system ensures excellent consistency, making it ideal for establishing high-quality and consistent cell banks.
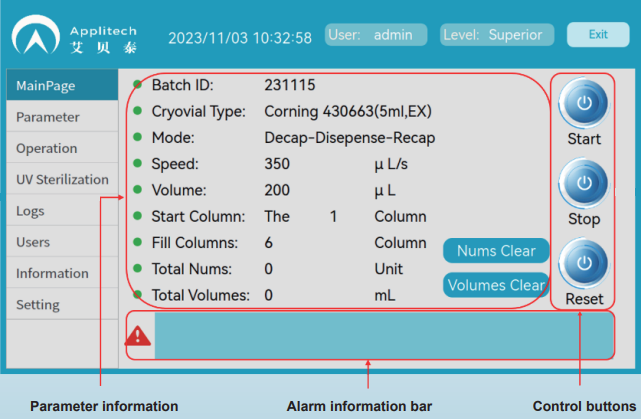
High-Definition Touch Screen with Intuitive Software Operation
▪ Customizable Filling Models: Select appropriate rotary heads, brackets, and trays based on the cryovial type.- "Decap – Dispense– Recap" mode
- "Dispense Only" mode
- "Decap only" mode
- "Recap Only" mode
▪ Filling Flow Rate: Fast filling speeds ranging from 350–800 μL/s, allowing the filling of ~1,000 cryovials in 30 minutes.
▪ Filling Volume: Dispensing volume range of 250 μL–5 mL with excellent consistency.
▪ Filling Rows: Cryovials can be loaded in multiples of 4 (4, 8, 12, 16, 20, or 24 vials) with the option to configure 1, 2, 3, 4, 5, or 6 rows.
▪ Cumulative Count and Volume: The software tracks the total number of cryovials filled and the cumulative filling volume in real time.
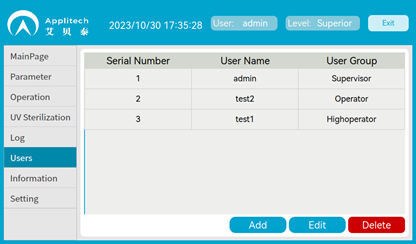
Audit trail, User Management
AbioFill
V100 is GMP compatible with audit trail and multi-level authority management
functions:
•
Account and password login
•
Multi-level user management
• Audit trails
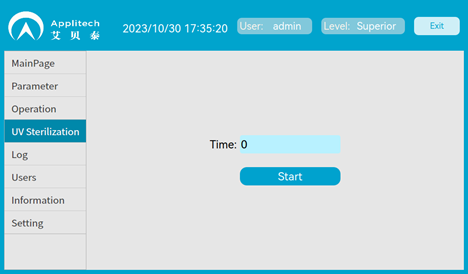
UV sterilization
Customized UV sterilization lamp is optional, and it is controlled by software to sterilize the filling module and the rack transfer unit.
Compatible with Various Cryovials

Applications
• Filling for High-Yield, Stable 3-Tier Cell Banks
3-tier cell banks provide certified, quality-consistent cells capable of sustained stable propagation for biophar maceutical production. The AbioFill V100 enables effortless large-scale cell filling through automated opera tions.
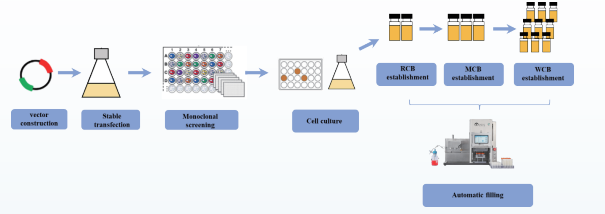
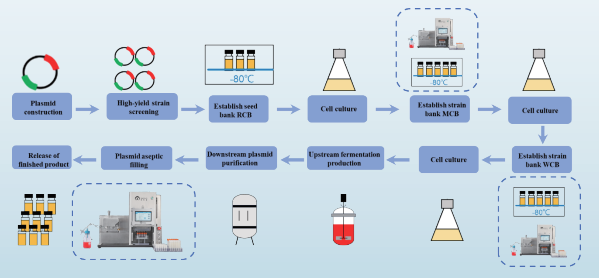
• Filling for 3-Tier Strain Libraries and cGMP-Grade Plasmid/Viral Vector Libraries
Plasmids are essential tools in cell, gene, and vaccine therapies. Establishing stable 3-tier strain libraries is critical for plasmid production. In gene and cell therapy, transient transfection technology is widely used, requir ing large quantities of GMP-grade plasmids. Additionally, in therapeutic drugs and vaccine research, plasmids can be directly introduced into the body as the final product to express target genes. The AbioFill V100 ensures efficient and sterile filling for 3-tier strain libraries and plasmid libraries.
• Cryovial Filling for Biopharmaceuticals, Such as Cytokines and Viral Fluids
Due to their biological activity, biopharmaceuticals require strict temperature control during storage, making the filling process especially critical. These products are typically stored in cryogenic containers, such as cryovials of various sizes, that match the dosing requirements.




