Company news
Company news
Optimization of Bioreactor Culture Process for Influenza B Vaccine Strain
In a collaborative effort, teams from the Chinese Academy of Agricultural Sciences and Shanxi Agricultural University published a study titled "Optimization of the Culture Process for Influenza B Vaccine Candidate Strain in MDCK Suspension Cells" in Virologica Sinica. The study focused on optimizing the bioreactor culture process for the selected rIBV strain in MDCK cells, resulting in a high-infectivity, high-viral titer MDCK cell-derived vaccine candidate strain rIBV. This lays the foundation for downstream influenza vaccine production and purification processes.
Seasonal influenza is an acute respiratory infectious disease caused by influenza viruses circulating globally. Currently, influenza B virus is among the main viruses affecting humans. Vaccination remains the most effective measure to combat influenza outbreaks in China. MDCK suspension cell culture does not require serum and offers better stability and safety compared to chicken embryo and Vero cell cultures. Bioreactors can provide a large number of synchronously dividing and rapidly proliferating cells, ensuring vaccine quality, making it the mainstream process for current vaccine production.
To mass-produce the recombinant influenza B vaccine candidate strain rIBV, researchers explored the optimal culture conditions for rIBV in serum-free MDCK cells, then scaled up to bioreactors to optimize process parameters. After 15 continuous passages, they measured the genetic stability and viral titer of the MDCK cell-derived strain rIBV. The optimal culture conditions for rIBV in MDCK cells were determined to be: Time of Infection (TOI) at 72 hours, Multiplicity of Infection (MOI) at 0.001, Cell Concentration at Infection (CCI) at 4.0×10^6 cells, TPCK trypsin concentration at 2 μg/mL, 50% dissolved oxygen (DO), and pH at 7.2±0.5. The rIBV strain maintained high genetic stability after 15 continuous passages in MDCK cells. Compared to the chicken embryo-derived rIBV strain, the rIBV strain adapted to MDCK cells showed an increase in EID50 from 10^6.25/mL to 10^9.5/mL and TCID50 from 10^4.5/mL to 10^8.25/mL after 15 passages.
MDCKs Cell Bioreactor Culture
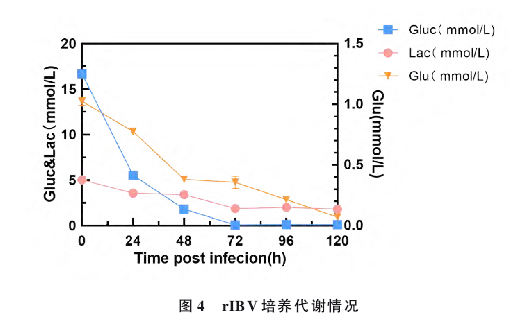
MDCKs cells were expanded stepwise using shake flasks. Healthy cells were then inoculated into a calibrated 5L bioreactor at an initial density of 1.5×10^6 cells, using a stirred culture method with an effective culture volume of 3L. The passage density followed the optimized culture conditions determined in the shake flasks. Other key parameters were: temperature at (37±0.1)℃, pH at 7.2±0.3, DO at 50%, and stirring speed at 100 rpm. The culture was maintained for 120 hours continuously. Every 12 hours, cell culture samples were taken to monitor cell density and viability, with three replicates. Samples were taken every 24 hours, processed according to the reagent kit instructions, to measure glucose, lactate, and glutamate content, and cell counting was performed with three replicates each time.
Optimal DO screening was also conducted
After stepwise expansion of MDCKs cells in shake flasks, healthy cells were inoculated into a calibrated 5L bioreactor at an initial density of 1.5×10^6 cells, using a stirred culture method with an effective culture volume of 3L. The relevant parameters followed the optimized culture conditions determined in the shake flasks. The key parameters were: temperature at (33±0.1)℃, pH at 7.2±0.05, and stirring speed at 120 rpm. DO was set at three levels for comparison: 30%, 50%, and 70%. Cell culture samples were taken every 24 hours to measure HA titer, with three replicates each time.
MDCKs cells were inoculated into the 5L bioreactor with key parameters set as follows: temperature at (33±0.1)℃, pH at 7.2±0.05, and stirring speed at 120 rpm. After determining the optimal DO level, 50% DO was established as the setting for subsequent bioreactor cultures.
Screening for Optimal pH
After stepwise expansion of MDCKs cells in shake flasks, healthy cells were inoculated into a calibrated 5L bioreactor at an initial density of 1.5×10^6 cells, using a stirred culture method with an effective culture volume of 3L. The relevant parameters followed the optimized culture conditions determined in the shake flasks. The key parameters were: temperature at (33±0.1)℃ and DO set according to the optimized value. pH was set at four levels for comparison: 6.8, 7.0, 7.2, and 7.4, with a deadband of 0.05. Stirring speed was set at 120 rpm. Cell culture samples were taken every 24 hours to measure HA titer, with three replicates each time.
MDCKs cells were inoculated into the 5L bioreactor with key parameters set as follows: temperature at (33±0.1)℃, DO at 50%, and stirring speed at 120 rpm. After determining the optimal DO, we selected a pH of 7.2 with a deadband setting of 0.05 for subsequent bioreactor cultures.
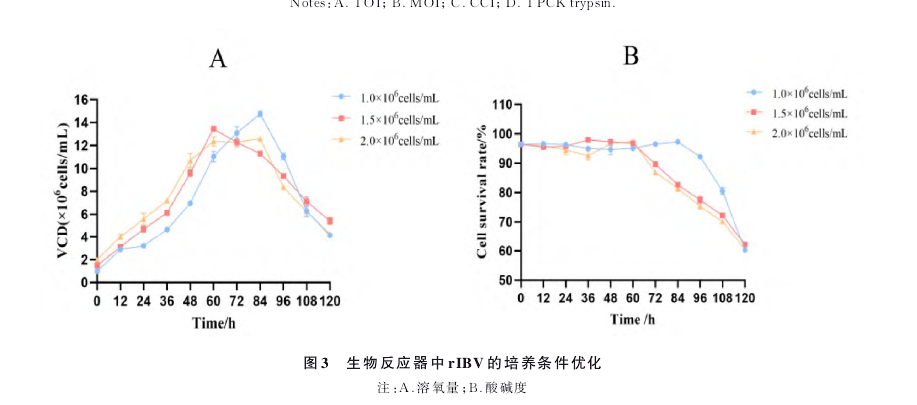
Results
After 15 continuous passages of the recombinant chimeric influenza B virus rIBV in MDCKs cells, a high-titer and genetically stable MDCKs cell-derived vaccine strain was obtained. We studied the virus's growth characteristics and genetic stability during the passage process. Following the optimized conditions in the bioreactor, a vaccine strain of rIBV was selected that is both genetically stable and exhibits high titer. The increase in virus titer further demonstrates the value of MDCKs cells as a substrate for vaccine production.
Summary
In this study, we optimized the proliferation conditions for rIBV in MDCKs cells and successfully scaled up to a 5L bioreactor. After continuous passage, we obtained a high-infectivity and high-titer MDCKs cell-derived rIBV vaccine strain, laying a solid foundation for the large-scale production of high-quality vaccines.
Bioreactor cultivation offers higher yields and more advanced control systems. The number of parameters that can be monitored and controlled depends on the sensors and control components in the bioreactor, enabling better screening and optimization of culture conditions. The process is straightforward, flexible, and highly applicable; it allows for easy scaling and provides a homogeneous environment for cell growth and proliferation. The product quality remains stable, making it well-suited for industrial production. Additionally, the simpler pipeline layout provides good sterility conditions, reducing the risk of contamination during cell growth.
Applitech has deep expertise in the biopharmaceutical field, with nearly 20 years of experience in the bioreactor industry. We are committed to developing high-end, self-designed bioreactors. Our team of over 150 researchers has meticulously developed the AbioBundle series of glass jar bioreactors. The AbioBundle M/E/P/D series, crafted with profound understanding of user needs and dedication, stands out with its exceptional design. We believe that the more you compare, the more professional it becomes. Applitech's self-designed bioreactors will provide strong support for your process development!






