Company news
Company news
[Cell Filling] AbioFill V100 Helps MSC Cell Filling Automation
The establishment of cell bank can provide qualified, same quality and stable cells for the production of biological products. Cell bank is the source of biopharmaceuticals and is the most important. For cell bank for production or verification, regulatory authorities in various countries generally require three-level management, namely original cell bank (PCB), master cell bank (MCB) and working cell bank (WCB). PCB is responsible for providing seed cells. After continuous cloning, screening and identification, MCB is formed, and then WCB is finally formed through the process of passage amplification, which is used for large-scale biological product production. If stably expressed cell lines or commercial cell lines are imported and purchased, secondary cell banks consisting of MCB and WCB can also be used for management. Of course, in some special cases, only MCB primary cell banks can be established, but they need to be approved by regulatory authorities, which shows their strict requirements for cell quality and drug safety.
The number of PCBs is calculated according to the actual situation, and each variety may be different. It is necessary to consider the subsequent production, verification needs, preservation period, number of tests, frequency of re-inspection, etc. The number of MCB and WCB frozen is determined according to the needs. It is necessary to ensure that the subsequent production and verification are sufficient, i.e., follow the "sufficient principle".
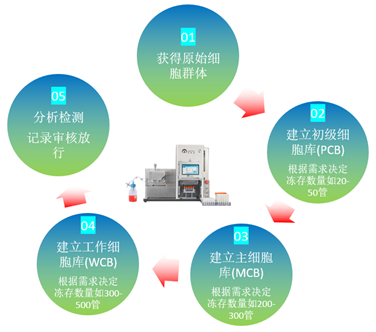
Cell bank establishment process
Stem cell therapy has proved to be a promising innovative therapy, in which mesenchymal stem cells (MSCs) are widely used due to their low immunogenicity, high proliferation potential and immunomodulatory properties. According to the latest literature statistics, as of 2023, there have been more than 1,000 registered clinical trials using MSC therapies worldwide, and 12 MSC therapies have been approved for commercialization by regulatory agencies. In the future, mesenchymal stem cells will play an important role in the treatment of more diseases. However, the cryopreservation of MSC cells is not easy, and the successful establishment of reliable MSC cell banks often requires a variety of operational details. In addition, MSC metabolic activity and adhesion ability decrease in ultra-low temperature environment, and cell membrane and cytoskeleton change. More commonly, MSC after cryopreservation, basically there is a decline in cell viability, which has a certain impact on clinical treatment effect.
MSC as seed cells or working cells, cell cryopreservation is an unavoidable step. As described in ICH Q5D, preventing contamination by exogenous agents, ensuring uniform distribution of cells in each cryopreservation tube, and ensuring that recovered cells have constant viability that meets production requirements are the three basic requirements for cell bank preparation. At present, most biopharmaceutical enterprises in China still use manual method to fill cells, which has some problems such as poor quality of cell bank caused by long contact time between cell samples and frozen solution, errors caused by a large number of manual repeated operations and risk of contamination. In order to save time for building a bank, improve cell consistency, reduce pollution risk and save cost of quality control, a reliable and effective cell filling method is needed. This article will focus on introducing AbioFill V100 cell filling system, a solution for high-throughput automated filling of MSC cells.
MSC cell filling experiment content
1. Experimental materials:
Cells: adherent mesenchymal stem cells (MSC)
Cryopreservation solution: 90%FBS+10%DMSO Cryopreservation solution: 90%FBS 10%DMSO
Cryotube: Corning 430659 (2ml,Ex)
AO/PI staining solution
Program Freezer Box, etc.
2. Equipment:
Filling System: AbioFill V100 Cell Filling System
Cell quality monitoring: Countleader cell counter FL1000
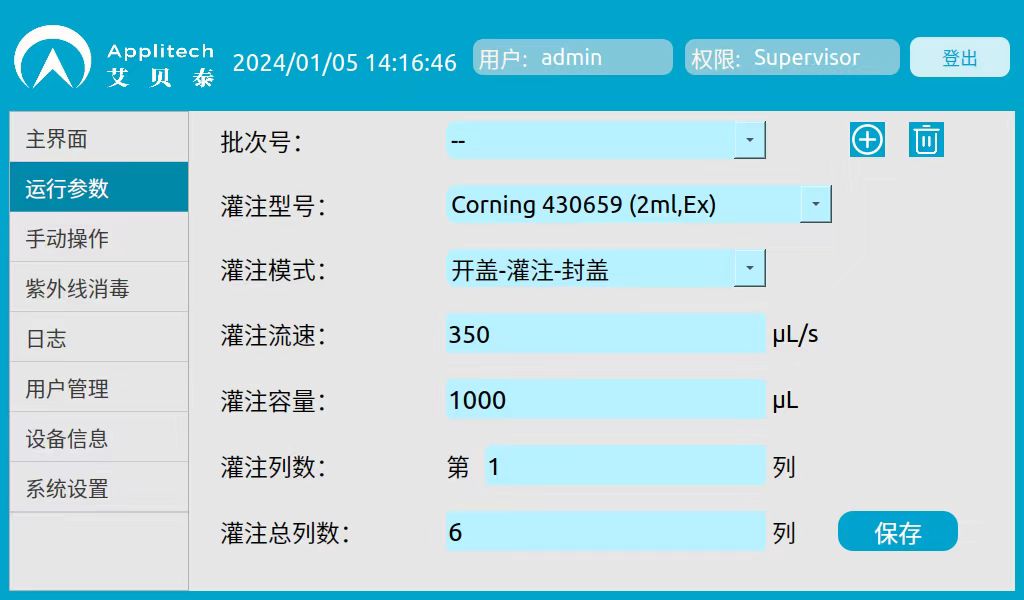
3. Experimental parameters:
Filling Mode: Open Cap-Fill-Cap
Perfusion flow rate: 350μL/s
Perfusion volume: 1000μL
5. Analysis and discussion of results:
MSC cells were resuspended in 30mL cryopreservation solution (50mL centrifuge tube system) before being put into the machine. Before filling, cell samples were reserved for cell counting. The cell viability was 97.55%, and the cell density was about 1.15E6 cells/mL. The filling system was used to fill 24 cryotubes at a volume of 1mL/tube, the cells of the 24 cryotubes were sampled for cell counting after filling, and the 24 cells were frozen at-80℃ using a programmed cooling box. After 24 hours of freezing, the cells were resuscitated in 37℃ water bath and counted.
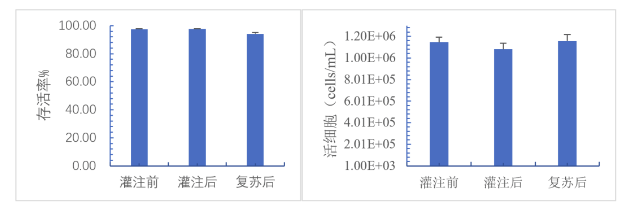
Bar graph of mean viability (left) and mean viable cell density (right) before/after perfusion and after resuscitation (n=24)
Average viability and viable cell density before/after perfusion and after resuscitation (n=24)
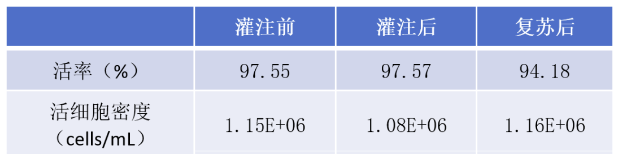
The statistical analysis showed that there was no significant difference between cell viability and cell concentration before and after perfusion, but the cell viability decreased slightly after resuscitation, the decrease range was about 3%, which was acceptable. The decrease of viability may be caused by the sampling and counting process after filling, which makes the cells stay in the freezing solution at room temperature for too long. Shortening the operation time can effectively avoid the decrease of viability.
The above experiments confirmed that AbioFill V100 automatic cell filling system can automatically and efficiently fill MSC cells, the quality of cells before and after filling is not affected, and can establish high-quality and homogeneous MSC cell banks, solve the industrialization problem of large-scale cryopreservation of MSC cells, and promote the good development of cell therapy industry.

AbioFill V100 Filling System

FL1000 Cell Counter
Scan the QR code to make an appointment
AbioFill V100 automatic filling system demo







