Company news
Company news
Bioprocess Membranes: The “Invisible Guardians” Behind Biopharmaceutical Manufacturing
Bioprocess Membranes: The “Core Code” Behind Technological Breakthroughs
Biopharmaceutical manufacturing demands far more stringent production environments than traditional chemical pharmaceuticals, and membrane materials are one of the core components that ensure these rigorous standards are met. Applitech’s domestically developed MC-BS006 bioprocess membrane is specifically designed for process development and production in the biopharmaceutical industry. It is suitable for various stages of the process, including the preparation of culture media and buffer solutions, intermediate formulation, storage, and bulk drug filling operations. Featuring a seven-layer co-extruded structure, this multilayer membrane offers enhanced physical resistance and is characterized by low extractables and leachables.
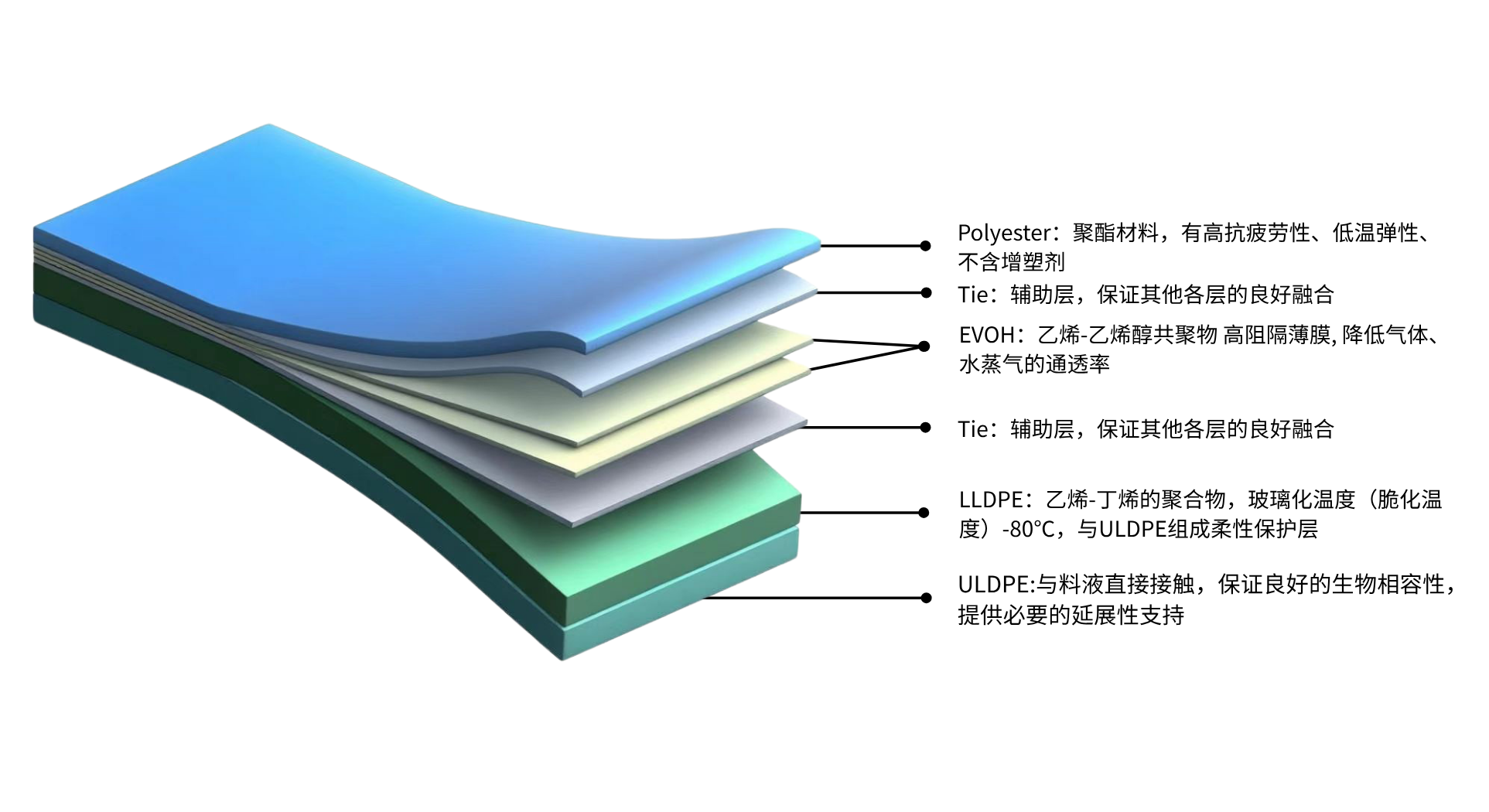
Figure 1. Structure and Features of Applitech MC-BS006
Stable Physicochemical Properties of MC-BS006 Before and After Irradiation
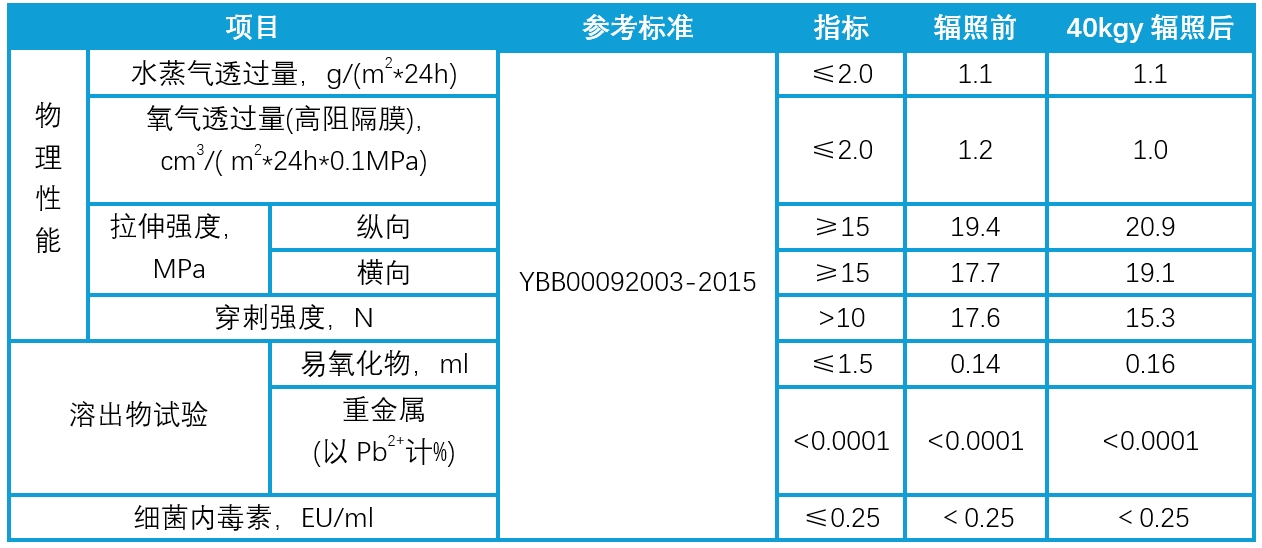
Table 1. Comparison of Physicochemical Properties
The biological safety of MC-BS006 has been validated in parallel with imported membrane materials, with comprehensive validation reports available upon request.
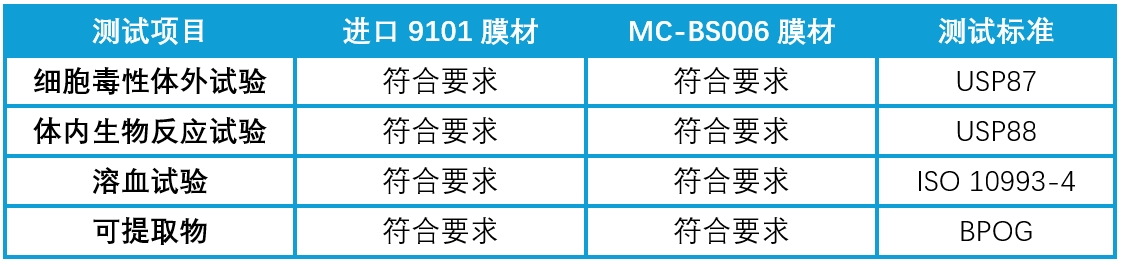
Table 2. Comparison of Biological Safety
MC-BS006 demonstrates excellent chemical compatibility, contributing significantly to the advancement of biopharmaceutical processes.
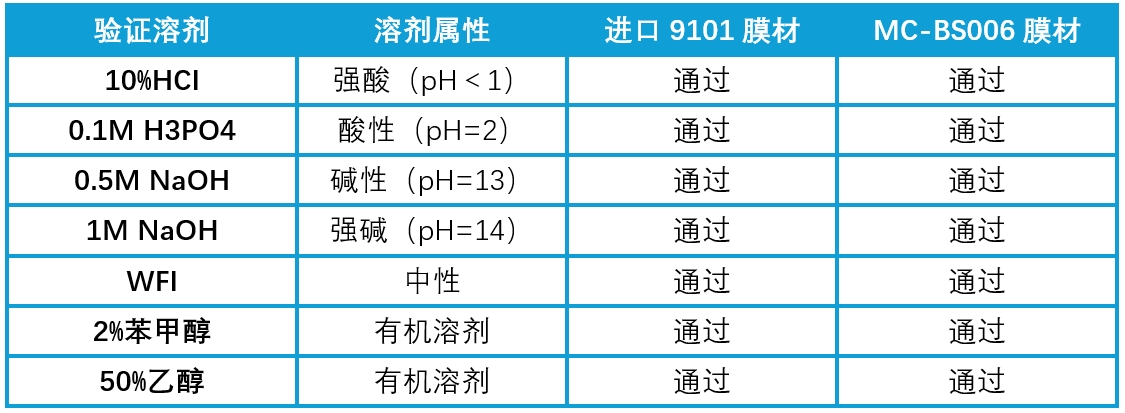
Table 3. Partial Comparison of Chemical Compatibility
In addition, Applitech’s domestically produced MC-BS006 bioprocess membrane benefits from a stable and secure supply chain. All raw materials are sourced from domestic suppliers, and both membrane manufacturing and bioprocess bag assembly are completed within the country, ensuring reliable support for the production operations of domestic biopharmaceutical companies.
Conclusion: Though Small, Membranes Define the Future
From the rapid mass production of antibody biologics to breakthroughs in the ADC industry, bioprocess membranes have always been the “unsung heroes” behind the scenes. With the acceleration of domestic substitution and continuous technological advancements, these “invisible materials” are poised to reshape the competitive landscape of the global biopharmaceutical industry.






