Company news
Company news
Solution for Single-Cell Cloning and Library Establishment of iPSC-derived Stem Cells
In recent years, an increasing number of iPSC-based therapies have entered clinical trial stages, offering new possibilities for disease treatment. The Technical Guidelines for Pharmaceutical Research and Evaluation of Human Stem Cell Products (Trial) clearly state that iPSCs, including those involving genetic modifications, must undergo single-cell cloning and the establishment of monoclonal cell lines, along with the construction of a robust cell bank. This requirement is not only a critical foundation for ensuring the quality of iPSC-related products, but also a key safeguard for the successful development of iPSC-based therapeutics.
iPSCs are highly sensitive to handling conditions and place stringent requirements on equipment. Applitech provides specialized instruments and services to support both upstream and downstream development of iPSC-based processes. Traditional single-cell sorting methods, such as fluorescence-activated cell sorting (FACS), may cause cellular damage, leading to reduced viability or loss of pluripotency. Meanwhile, limiting dilution offers low efficiency and often fails to meet the demands of iPSC development.
Gentle sorting technologies help preserve the genetic stability of cells. During the sorting process, cells are exposed to minimal mechanical stress and chemical damage, thereby reducing the risk of genetic mutations or chromosomal abnormalities. This is crucial for establishing high-quality monoclonal cell banks, as genetic stability is a key requirement for compliance with GMP standards and for clinical applications.

The single-cell isolation and selection system developed by Cytena (Germany) utilizes a patented microfluidic technology to efficiently and gently isolate individual iPSCs into 96- or 384-well plates. Each dispensing event is documented with nozzle imaging to verify single-cell origin, which facilitates regulatory documentation and application submission.
Rapid single-cell isolation: 1–2 minutes per 96-well plate
-
Ultra-small droplet volume (~200 pL) with >95% single-cell accuracy
-
Gentle sorting process ensures no cell damage and high monoclonality
-
Disposable sterile chips eliminate cross-contamination between projects
AbioFill V100 Cell Filling System
With the growing application of iPSC technology in cell therapy and regenerative medicine, there is an increasing demand for larger and higher-quality cell banks. Automated filling systems enable efficient large-scale aliquoting of cells while minimizing potential damage to iPSCs during the filling process.

Applitech’s independently developed automated filling system enables the efficient establishment of a three-tiered iPSC cell banking system, providing a consistent and reliable source of high-quality iPSCs for downstream differentiation processes
- Automated operation enables easy large-scale cell aliquoting
- High efficiency and speed: nearly 1,000 vials can be filled within 30 minutes
- High consistency with filling volume accuracy within ±5%
- Supports three-tier cell banking and reference standard filling
- Compliant with GMP requirements, featuring audit trails and access control
Countleader FL 2000 Cell Counter
During the induced differentiation of iPSCs, cell concentration plays a critical role in differentiation efficiency, quality, and therapeutic dosing. Excessively high concentrations may cause cell aggregation, negatively affecting differentiation outcomes, while overly low concentrations may prevent effective interaction with differentiation-inducing factors. Therefore, a high-precision cell counter is essential to accurately measure cell numbers, ensuring that cell concentrations during differentiation meet experimental design requirements. This also provides precise dosing information for cell therapies, guaranteeing batch-to-batch consistency in therapeutic quality
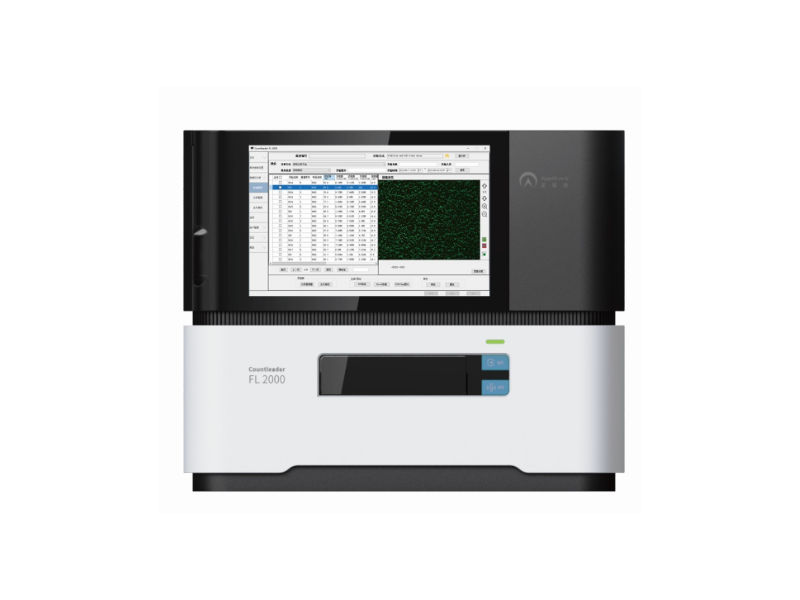
- Consumables pre-embedded with AOPI dye enable automatic staining, reducing human error
- Rapid analysis: 17 minutes for 24 samples, improving workflow efficiency
- 24-channel system supports high-throughput simultaneous sample analysis
- Diameter ratio analysis helps monitor iPSC differentiation status
- Software displays fluorescence parameters for each individual cell
- Growth curve plotting capability to track cell proliferation trends
- Integrated design with a 12-inch HD touchscreen for intuitive and convenient operation






