Company news
Company news
Process Evolution: The Iterative Transformation from Traditional Cultivation to Intensified Processes
As the core of upstream biomanufacturing, the intensification of cell culture is key to optimizing the overall bioprocess flow. By shortening the cell growth phase or reducing the production time of N-stage bioreactors, the manufacturing process can be accelerated. Currently, upstream cell culture primarily utilizes batch culture, fed-batch culture, perfusion culture, and continuous culture. Among these, continuous culture based on perfusion technology has demonstrated significant advantages over traditional batch and fed-batch cultures.
Process intensification through perfusion culture has been widely applied to the production of unstable and low-yield proteins, primarily in small-scale operations. Despite its outstanding performance in small-scale production, the large-scale commercial application of perfusion technology still faces numerous challenges, mainly in the complexity of process development, the high demands for automation control, and cost management difficulties.
In recent years, an attractive and highly efficient platform process that has gained strong momentum is the intensified or high-inoculation-density fed-batch bioreactor. This approach features an inoculation density that is 20 times higher than that of traditional fed-batch bioreactors, achieved by utilizing perfusion culture in the N-1 stage to generate a highly concentrated seed culture. This process shifts the burden of cell growth to the N-1 stage while allowing the N-stage bioreactor to maintain a high cell density.
Since the total yield of monoclonal antibodies is directly correlated with the total number and viability of cells in the culture, an increase in cell density translates into a significant enhancement in the overall productivity of the bioreactor. Over the past decade, numerous examples of this manufacturing process have emerged. Compared to low-density bioreactors, those inoculated with 10 × 10⁶ cells/mL have demonstrated a 100% increase in titer.
In the article "Biomanufacturing evolution from conventional to intensified processes for productivity improvement: a case study," published in mAbs in May 2020, Bristol-Myers Squibb (BMS) shared a case study of productivity improvement through the transition from conventional to intensified processes. The study demonstrated the comparison of three processes for producing monoclonal antibodies using CHO cells: traditional fed-batch process (Process A, 1000L scale), N-1 intensified fed-batch process (Process B, 1000L scale), and N-1 perfusion intensified fed-batch process (Process C, 2000L scale).
In Process C, the N-1 perfusion technology was applied in the upstream phase, and downstream processes were enhanced with multi-column chromatography (MCC) and integrated polishing steps, significantly improving production efficiency. This process demonstrated excellent scalability and effectively reduced production costs through process optimization, laying a solid foundation for the future realization of fully continuous production.
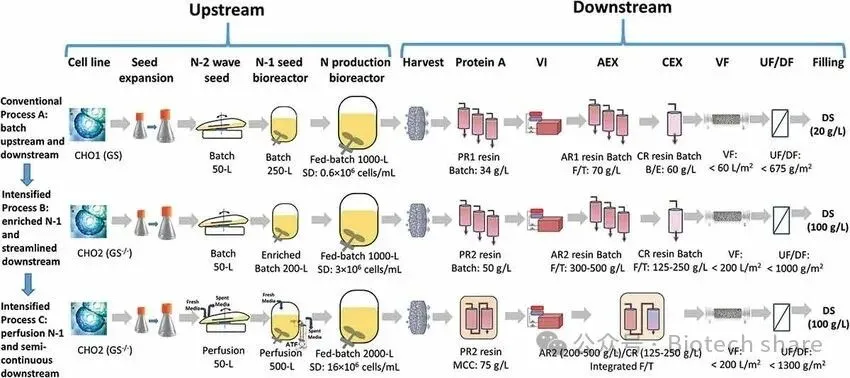
Note: Process A uses traditional fed-batch culture. Process B improves the cell inoculation density in the N stage by using batch or fed-batch culture in the N-1 stage. Process C enhances the cell inoculation density in the N stage by introducing perfusion operations in the N-1 seed culture stage.
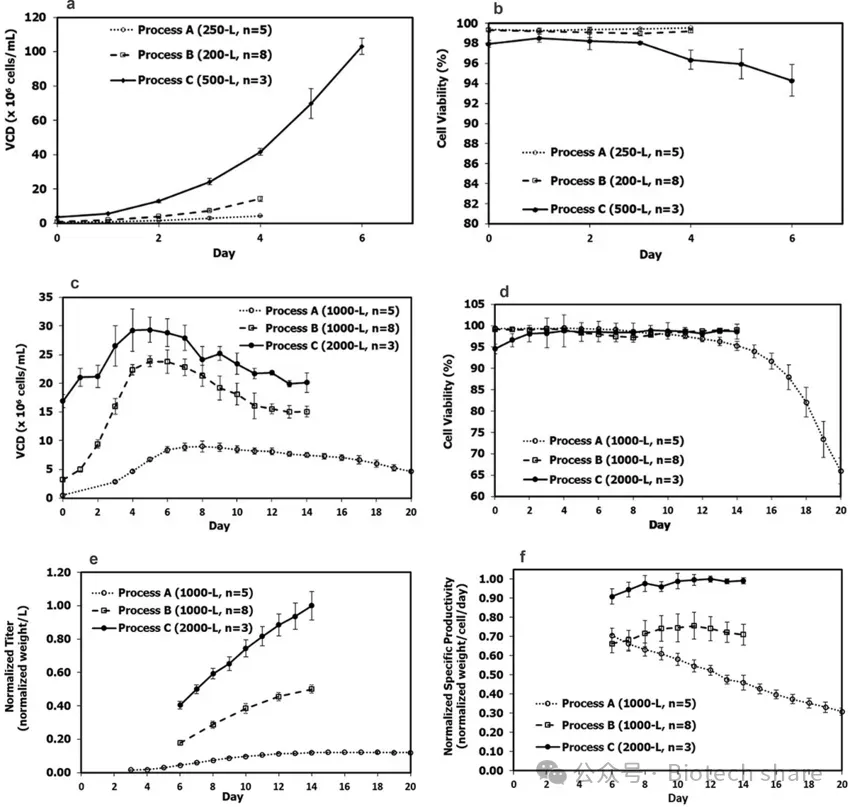
The authors compared the viable cell density (VCD), cell viability, and subsequent fed-batch production performance of seed culture in the N-1 stage under different processes. The results showed that, in the 200L to 500L scale, the VCD of the traditional fed-batch process (Process A) was 4.29±0.23×10⁶ cells/mL. In contrast, the intensified processes B and C showed significant increases in VCD, reaching 14.3±1.5×10⁶ cells/mL and 103±4.6×10⁶ cells/mL, respectively. Particularly, Process C exhibited a significant positive effect of perfusion technology on cell expansion (Figure a). Although there was little difference in cell viability between processes A and B, Process C showed a slight decrease in cell viability, which did not significantly affect the subsequent production culture (Figure b).
In the 1000L to 2000L scale, the highest VCD in Process C reached 29.3±2.19×10⁶ cells/mL, far higher than those of Processes A and B (Figures c and d), indicating that Process C also performed excellently in large-scale cultivation. Furthermore, the standardized yield and cell-specific productivity of Process C were significantly higher than those of Processes A and B (Figures e and f), with increases of 8 times and 2 times, respectively, compared to Process A and B. This demonstrates that the introduction of the N-1 intensified process greatly enhanced production efficiency.
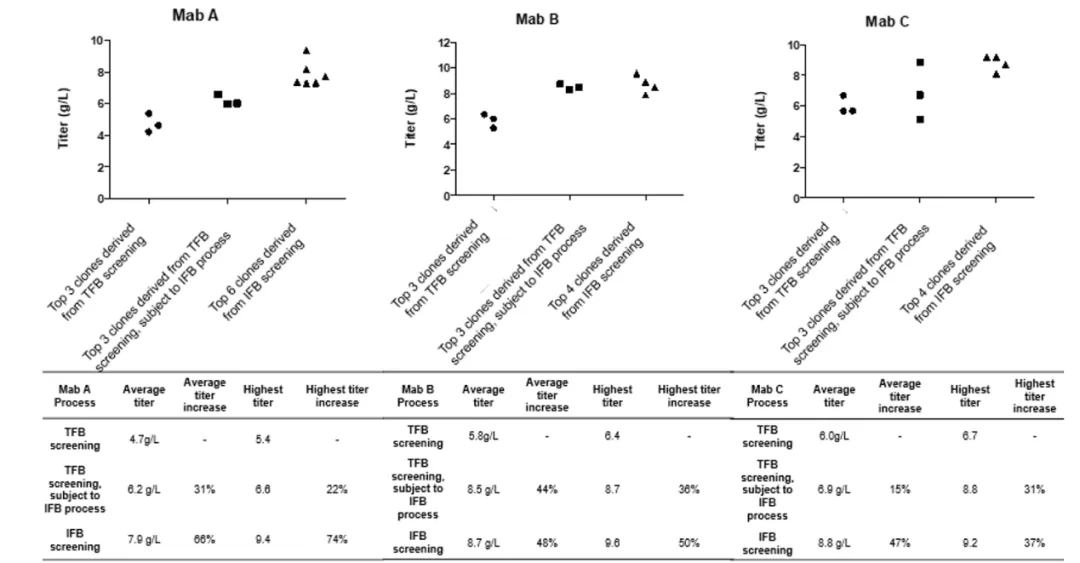
A recent study titled "Establishment of a high-throughput scale-down clone screening platform for intensified fed-batch culture of CHO cells" published in Biotechnology Progress also highlighted the significant improvement in yield using the IFB platform compared to the TFB platform. In the experiment, three monoclonal antibodies (mAb A, B, and C) were used. The results indicated that, compared to the traditional fed-batch (TFB) process, the IFB process was able to significantly increase the yield, with the highest reaching up to 9.6 g/L.
In the biopharmaceutical industry, improving productivity has always been a core goal of research and development, with processes continuously iterating and upgrading. IFB (Intensified Fed-Batch) achieves higher inoculation density through perfusion culture in the N-1 seed stage, resulting in higher yields. However, this intensified strategy also presents several challenges. In the later stages of cell culture, cells often face rapid declines in viability and reduced product synthesis capability due to the rapid accumulation of secondary metabolites and the depletion of key nutrients.
Recently, WuXi Biologics independently developed the Intermittent-Perfusion Fed-Batch (IPFB) process. This process incorporates one or more intermittent perfusion operations during the IFB production process. This allows for the timely removal of cell culture media enriched with secondary metabolites, while fresh nutrients are introduced, helping to better maintain cell health and significantly improve yields. Additionally, by using an ATF (Alternating Tangential Flow) perfusion system with smaller pore sizes (50 kD), the product is retained in the reactor, enabling a single harvesting operation at the end of production. Compared to IFB, the IPFB model can increase yields by an average of 50%.






